|
Солитарные фиброзные опухоли плевры: клинические характеристики, хирургическое лечение и исходы
Eur J Cardiothorac Surg 2002;21:1087?
Solitary fibrous tumors of the pleura: clinical characteristics, surgical treatment and outcome
Pierre Magdeleinata*, Marco Alifanoa, Antonio Petinoa, Jean-Philippe Le Rochaisb, Elisabeth Dulmetc, Françoise Galateaud, Philippe Icardb, Jean-François Regnarda
a Service de Chirurgie Thoracique, Hôtel-Dieu, 1, Place du Parvis Notre Dame, 75181 Paris Cedex 04, France
b Department of Cardio-Thoracic Surgery, CHU Côte de Nacre, Caen, France
c Unit of Pathology, Marie Lannelongue Hospital, Le Plessis Robinson, France
d Unit of Pathology, CHU Côte de Nacre, Caen, France
Received 8 November 2001; received in revised form 24 January 2002; accepted 7 February 2002.
* Corresponding author. Tel.: +33ם? fax: +33ם?
e-mail: pierre.magdeleinat@htd.ap-hop-paris.fr
Abstract
Objective: The aim of this paper is to study clinical characteristics, surgical treatment and outcome of patients with solitary fibrous tumor of the pleura operated in our institutions in a 20-year period. Methods: Clinical records of all patients operated for solitary fibrous tumors of the pleura between 1981 and 2000 were reviewed retrospectively. Tumors were classified as malignant in the presence of at least one of the following criteria: (1) high mitotic activity; (2) high cellularity with crowding and overlapping of nuclei; (3) presence of necrosis; (4) pleomorphism; otherwise they were considered as benign. Results: Sixty patients (mean age 55 years) were operated in this period. None had asbestos exposure. Symptoms were present in 31 cases. Surgical approaches included thoracotomy (n=53), video-assisted thoracoscopy (n=6), and median sternotomy (n=1). Tumors originated from visceral pleura in 48 cases, from parietal, mediastinal or diaphragmatic pleura in seven, two and three cases, respectively; their mean diameter was 8.5 cm. Tumors could be resected with their implantation basis in 49 patients. In the remaining 11, extended resections were performed, including lung parenchyma (lobectomy, n=4, pneumonectomy, n=2), osteomuscular chest wall structures (n=2), diaphragm (n=2), and pericardium (n=1). Two postoperative deaths (due to myocardial infarction and pulmonary embolism, respectively) occurred. Tumors were pathologically benign in 38 cases and malignant in 22 cases. Mean follow-up was 88 months. Resection was complete in all the patients with benign tumors and no recurrence occurred. Resection was considered as complete in 21/22 malignant tumors. Local recurrence was observed in two cases. Both could be successfully managed by iterative exeresis (no extended resection had been initially performed). Metastatic disease (responsible for patient’s death) was observed following the only incomplete resection. Actuarial 5- and 10-year survival rates were 97% for benign tumors and 89% for malignant ones. Conclusions: Surgical resection provided cure in all the patients with benign tumors. As insufficiency of exeresis is associated with all recurrences in malignant tumors, completeness of resection is in our experience the best prognostic factor in these forms.
Key Words: Pleura • Solitary • Fibrous tumor • Surgery • Prognostic factors
1. Introduction
Localized fibrous tumors of the pleura (LFTP) represent a rarely encountered clinical entity [1㪤]. After several decades of controversies, it is nowadays recognized that they originate from mesenchimal cells of submesothelial tissue of the pleura [1,2,4]. It is recognized that surgery represents the treatment of choice of these tumors [1,2,5,11]. Criteria for distinguishing pathologically benign from malignant forms have been established and are widely accepted [1,2]. It has been reported that local recurrence may occur following complete exeresis of benign LFTP [1,5]; on the other hand, complete exeresis would offer possibility of cure in a considerable percentage of cases with malignant LFTP [1,2,5]. Due to the rarity of LFTP, such opinions are based on relatively small series of patients [3,5,6,11]. In the present work, we report our experience with management of patients with LFTP in a 20-year period, in order to study their clinical characteristics, treatment modalities and outcome.
2. Methods
We retrospectively reviewed clinical records of all patients who underwent surgery for resection of LFTP in a 20-year period (1981?). The same surgical team operated on 45 patients at the Marie Lannelongue Hospital up to August 2000 and at the Hôtel-Dieu Hospital thereafter; the remaining 15 patients were operated by the other surgical team at the Caen University Hospital. In all the cases, microscopic slides were re-examined; tumors were re-classified as benign or malignant according to criteria reported by England et al. [1]: (1) high mitotic activity (>4 mitosis/10 high-power fields, i.e. magnification= x400); (2) high cellularity with crowding and overlapping of nuclei; (3) presence of necrosis; (4) pleomorphism. Tumors were considered as malignant if at least one criterion was present.
In all the patients, preoperative evaluation included their history, physical examination, routine blood tests, standard chest X-ray, and thoracic CT scan. Fiber optic bronchoscopy was performed in all smoking patients and in case of proximally located lesions. Electrocardiography, spirometry and arterial gas analysis were routinely carried out. Perfusion lung scan was employed if a lung function impairment (forced expiry volume (FEV1<80%) of predicted value) existed. Echocardiography was carried out if a history of cardiovascular disease was present or if a pneumonectomy was anticipated. Isotopic myocardial scan was performed in patients with a history of ischemic heart disease.
Postoperative radiotherapy or chemotherapy was performed under the care of referring physicians, so no uniform protocol was employed.
Operative mortality was calculated by taking into account all the deaths occurring within 30 days from the operation or during the hospitalization. After completion of the study (February 2001), information about the health status of patients was obtained by the referring physicians or/and by the patients themselves. Percentage comparisons were made by the continuity-corrected 2 test. Survival rates, including non-cancer related deaths, were calculated by the actuarial method and compared by the log — rank test. Results were considered significant if the P value was less than 0.05. The BMPD statistical software [13] was employed.
3. Results
Sixty patients were operated for a solitary fibrous tumor of the pleura in the studied period. There were 25 men and 35 women; mean age was 55 years (range 30㫯). A smoking history was present in 25 cases; no patient had asbestos exposure.
3.1. Symptoms
Thirty one patients (52%) presented symptoms related to pleural fibroma: chest pain (n=15), cough (n=10), dyspnea (n=4), fever (n=2), more than 10% body weight loss (n=2). Hypertrophic osteoarthropathy and symptomatic hypoglycemia were present in four and one cases, respectively. Symptoms were present on the average for 13 months. Twenty nine patients were totally asymptomatic and pleural fibroma was discovered on a chest X-ray performed for other reasons.
3.2. Preoperative studies
In all the patients, the pleural tumor was evident on standard chest X-ray. Left or right hemithorax was involved in 31 and 29 cases, respectively. In 25 patients, the lesion was present in chest X-rays performed previously (5�� months, mean 68 months); in these cases, the lesion had been judged benign and a follow-up decided by the treating physicians. These patients had been eventually referred to us for surgery for apparition of symptoms or for a remarkable increase in size of the lesion.
Preoperative CT scan was carried out in all the patients. Mean main diameter of the lesions was 8.5 cm (range 1㪻). A moderate pleural effusion was associated with seven patients (in all the cases, the main diameter of the tumor was >8 cm). Forty eight patients underwent fiber optic bronchoscopy. No abnormalities were found in 39 cases. An extrinsic compression was present in nine cases: all of them had tumor >5 cm in its main diameter. CT-guided aspiration biopsy had been performed in 11 patients under the care of referring physicians before hospitalization in our centers; a preoperative histologic diagnosis had been obtained in five of them.
3.3. Treatment
Resection was carried out through a thoracotomy in 53 cases (postero-lateral, n=48; antero-lateral, n=5), a video-assisted thoracoscopy in six cases, and a median sternotomy in one case. In this last case at preoperative work-up, the lesion was considered as a probable thymoma.
In all the cases, a single lesion was found at surgical exploration. The tumor originated from the visceral pleura in 48 cases (80%); among them, a pedicle was present in 38 cases, whereas 10 tumors had no pedicle (sessile tumors). In seven cases, the lesions originated from the parietal pleura, in three from the diaphragmatic pleura and in two from the mediastinal pleura; all these tumors were sessile. Fibrous adhesions to adjacent structures were observed in 36 cases (lung, n=22; mediastinal pleura, n=5; diaphragm, n=5; and parietal pleura, n=4). Tumor was macroscopically encapsulated in 53 patients (88%), whereas a macroscopically invasive behavior was evident in the remaining seven cases.
All the patients underwent tumor exeresis. In 49 patients, tumor was resected with its implantation basis: this basis was resected ‘en bloc’ with the tumor either by a lung wedge resection (in the case of lesions originating from visceral pleura, n=42) or by a pleural excision (in the case of lesions originating from the parietal, mediastinal or diaphragmatic pleura, n=7) In 11 patients, extended resections ‘en bloc’ with the tumor were performed: in six patients, a formal lung resection (lobectomy, n=4; pneumonectomy, n=2) was necessary in the presence of voluminous tumors arising from the visceral pleura and extending deeply in the lung parenchyma; in five other patients with macroscopically invasive tumors ‘en bloc’ resection of surrounding structures was carried out (osteo-muscular chest wall structures, n=2; diaphragm, n=2; and pericardium, n=1).
Frozen sections were required when even a minimal doubt about adequateness of resection margins existed (especially in the case of sessile tumors, in order to perform in every case a wide surgical excision. So frozen sections were not required to distinguish benign from malignant tumors.
Postoperative radiotherapy was administered in one patient after complete resection of a giant malignant tumor; postoperative chemotherapy was administered in another patient after incomplete resection of a malignant lesion.
3.4. Pathologic examination
There were 38 (63%) benign and 22 (37%) malignant tumors. Details about malignity criteria are reported in Table 1. Seven out of 22 malignant tumors invaded one or more adjacent structures: lung (n=3), chest wall (n=2), diaphragm (n=2), and pericardium (n=1). In all the cases (n=7) with a macroscopically invasive behavior, malignancy was confirmed at pathology; otherwise, the absence of a macroscopically invasive behavior did not rule out malignancy, as 15 patients had a macroscopically well-encapsulated tumor that proved to be malignant at pathology. Comparison of clinical and anatomical characteristics of benign and malignant tumors is reported in Table 2. Resection was complete (R0) in 59 cases. It was microscopically not complete (R1) in one case, in the presence of a voluminous malignant tumor (main diameter=10 cm) invading the diaphragm. The lateral edges of diaphragmatic invasion were underestimated intraoperatively.
Table 1. Malignity criteria among the 22 malignant tumors
Table 2. Comparison of clinical and anatomical characteristics of benign and malignant tumors
Cyto-pathologic examination of pleural effusion found in seven cases at surgery always showed the absence of malignant cells.
3.5. Outcome
Postoperative course was uneventful in 56 patients (93%). In four cases, complications occurred: pulmonary embolism (n=1), myocardial infarction (n=1), persistent air leak (n=1), and transient ischemic attack (n=1). These complications were responsible for two deaths (operative mortality=3.3%); the two causes of deaths were acute myocardial infarction and massive pulmonary embolism; they occurred after exeresis of a voluminous (10 cm) benign tumor and of a giant (35 cm) malignant tumor, respectively.
Among the 58 patients who survived the postoperative period, mean follow-up was 88 months (range 3�� months). No patient was lost at follow-up.
After completion of the study, 55 patients were alive and disease-free. There were three late deaths, one of them was tumor-related. Overall 5- and 10-year survival rates were 94% (Fig. 1 ).
Fig. 1. Actuarial 5- and 10-year survivals of the overall population, and according to the presence or absence of malignity criteria.
Mean follow-up of patients with benign tumors (n=37) was 91 months (range 3��). No recurrence occurred; one late death (due to unrelated cause) was observed. Actuarial 5- and 10-year survival rates were 97% (Fig. 1).
Mean follow-up of patients with malignant tumors (n=21) was 80 months (range 23��). Three recurrences occurred (Table 3). Two local relapses were re-operated (Table 3, cases no. 1 and 2; Fig. 2 ); a complete exeresis of recurrences was achieved and both patients are alive and disease-free with a follow-up of 12 and 108 months, respectively. One metastatic recurrence (disseminated liver metastases, Table 3, case no. 3) occurred; it was responsible for patient’s death 32 months after the incomplete exeresis of the primary tumor followed by postoperative chemotherapy. One non-tumor related death was also observed; it occurred 25 months after surgery (complete exeresis) followed by postoperative radiotherapy. Actuarial 5- and 10-year survival rates of patients with malignant LFTP were 89% (Fig. 1).
Table 3. Pathological characteristics, surgical management and outcome of the three malignant recurrent tumors
Fig. 2. A case of local recurrence after exeresis of a malignant LFTP is illustrated. Chest X-ray of the initial LFTP involving visceral pleura of right lower lobe (right). CT scan showing the recurrent tumor at the same location 103 months later (left).
4. Discussion
LFTP represent a rarely encountered clinical entity, with few studies dealing with more than 25 patients [1,2]. It is well known that these tumors have often an indolent clinical course thus being asymptomatic for several years [5,6]: in 42% of our patients, the tumor was known for a long time (68 months on the average) prior to referral. On the other hand, there are no specific symptoms and preoperative diagnosis is extremely difficult or not possible in many cases. Hypertrophic arthropathy or hypoglycemia (that has been reported to occur in up to 25 and 4% of cases, respectively, in the literature [9,12]) may help in establishing diagnosis; in our experience they occurred only in 4 and 2% of patients, thus suggesting the extreme variability in the incidence of these symptoms. Thoracic CT scan shows in most cases a well-circumscribed round tumor with a homogenous density. However, these findings lack specificity [2,7] and other imaging findings are possible. Thus CT scan cannot differentiate benign from malignant LFTP. Difficulties in differentiating these tumors from others originating from mediastinum (Fig. 3 ) or chest wall (Fig. 4 ) are possible. Furthermore if the lesion is not homogeneous (Fig. 5 ), the differential diagnosis with a bronchogenic carcinoma may be also more difficult, especially in the presence of a smoking history (as in 48% of our patients). Nuclear magnetic resonance may provide some help, showing the fibrous character of the lesion [7]. In agreement with others [2,5,6], we found that diagnostic accuracy of CT-guided aspiration biopsy is not satisfactory (it was 45% in our series).
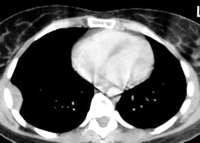
Fig. 3. A case of LFTP arising from visceral pleura of the mediastinal aspect of left upper lobe simulating a mediastinal tumor.
Fig. 4. A case of LFTP arising from visceral pleura of the right lower lobe simulating a chest wall tumor.
Fig. 5. A case of LFTP arising from visceral pleura of the left lower lobe. Note the heterogeneity of the lesion.
Surgery allows establishment of a definitive diagnosis as well as cure of the disease. It is well known that treatment of LFTP is based on surgical exeresis [1,2]. In our opinion, every suspected or proven LFTP must be operated because clinical and imaging criteria are unable to distinguish accurately benign forms from malignant ones (Table 2). Furthermore aggressive histological features may appear in the evolution of these tumors, as observed in one of our recurrent tumors (Table 3, case no.1). The completeness of the exeresis represents the primary objective of surgery [1,2]. Exeresis is performed through a thoracotomy or a video-assisted thoracoscopy. In our series, thoracotomy was carried out in 88% of cases. Video-assisted thoracic surgery may represent a valid option because of advantages in terms of postoperative pain, reduced respiratory impairment [14] and cosmetic results. Furthermore this approach can avoid an unproductive sternotomy (as we did once in our series) in case of doubt with a mediastinal tumor. However, the need of an incision to take out the operative specimen is related with tumor size. In our experience, we employed this approach in six cases (always tumors <5 cm). We evaluate systematically the possibility of a video-thoracoscopic approach in this kind of patients since 1991. In the experience of Suter et al. [5], video-assisted thoracoscopy was never employed for resection, whereas Rena et al. used video-assisted thoracoscopy in one out of 21 cases [11]. In the experience of Cardillo et al. [2], video-assisted thoracoscopy was employed more frequently: the tumors could be removed by VATS in 39 out of 55 cases; however, it must be considered that this series deals with patients operated after 1990. Thus we recommend video-assisted thoracoscopy in the presence of lesions <5 cm. Conversion to open approach should be performed in the case of difficulty of obtaining large free margins by the video-assisted technique.
Regardless of surgical approach, exeresis of LFTP is generally easy, especially in the presence of a pedicle. Large tumors with an invasive behavior may be more difficult to extirp and enlarged exeresis may be necessary. Generally in the case of voluminous sessile tumors involving deeply or largely adherent to the lung (inverted fibromas) a formal lung resection is necessary: this kind of tumor occurred in six out of 60 patients in our series. En bloc chest wall resection or exeresis of diaphragm, parietal pleura and pericardium may also be necessary to achieve a complete resection. Similar results were reported by Cardillo et al. [2] who performed two lobectomies, one bilobectomy and one pneumonectomy among their 55 patients, whereas Suter et al. [5] reported two lobectomies among their 15 cases and three chest wall resections among their 15 patients with LFTP. On the other hand, in the recent experience of Rena et al., including 21 patients, no ‘extended resection’ was necessary [11].
Peri-tumoral inflammatory adhesions are a frequent feature in these tumors (60% of cases in our series). In rare instances, these adhesions may be microscopically tumoral and consequently underestimated at surgical exploration: this probably explains the only case of incomplete resection in our series. Furthermore insufficiency of the exeresis probably explains the local recurrence of two malignant tumors: both were sessile tumors and had been treated by resection of tumor with its implantation basis (the resection had been considered as complete at pathological examination). So in agreement with others [2] we recommend in all the cases an exeresis with large free margins and the use of frozen sections when doubts about adequateness of margins exist: every LFTP must be viewed as possibly malignant.
In our series, diagnosis of malignity was established on the basis of criteria suggested by England et al. [1]. These criteria are currently widely accepted and have been employed in the most recent surgical series [2,5,6,11]. Their usefulness is also suggested by the American Registry of Pathology [15]. In our experience malignant forms of LFTP accounted for 37% of all cases. This percentage is similar to that observed in several other series (36% in the experience of England et al. [1], 38% according to Rena et al. [11], 30% in the experience of de Perrot et al. [6]), but quite different as compared to other ones (7% according to Cardillo et al. [2], but 60% in the experience of Suter et al. [5]). This variability could be probably justified either by the heterogeneity in studied populations or by relative subjectivity in the recognition of some pathology criteria, especially hypercellularity and pleomorphism. It should also be considered that the absence of the four malignity criteria (thus permitting to consider a tumor as benign) may be difficult to establish in the whole of a voluminous or a giant tumor, due to the frequent heterogeneity of these lesions. So careful pathological examination is mandatory to affirm the benignity of these tumors.
The best prognostic criterion is the completeness of resection [1,2,11]. In these cases, prognosis of LFTP is generally very satisfactory: in our experience, all the patients with benign LFTP had complete resection and no recurrence was observed. This result is in complete agreement with those of others [2]; however, occasional recurrences have been reported in other studies [1,6]. In particular, in the collected series of England et al. [1], a small percentage (1.4%) of benign LFTP recurred [1]. The reasons for the possible recurrence of benign LFTP after pathologically complete resections have not been established so far: it is possible to hypothesize that an insufficient resection may be the cause. Furthermore, as already outlined, the affirmation of benignity of a tumor may be sometimes difficult, especially in the presence of large or giant lesions. In our series isolated (without metastatic spread) local recurrence was observed in two out of 21 patients with malignant LFTP. This percentage is similar to that observed by other authors [2,11], but lesser that that reported in other series [1,5]. As outlined in previous experience [9], it is possible that earlier or later detection, different pathologic definition and differences in surgical technique may, at least in part, explain such differences.
A long follow-up is mandatory because of the possibility of late recurrence of these slow-growing tumors. In our, as well as in the experience of others [3,11], local recurrence could be successfully managed by redo surgery, thus suggesting that aggressive surgical management is justified in such cases.
In agreement with others [2] we found no data about the possible benefit of adjuvant radiotherapy or chemotherapy after resection of malignant tumors. In our retrospective series, only one patient received postoperative radiotherapy and another one received postoperative chemotherapy. It must be remembered that indication for postoperative treatments was established by the referring physicians. No conclusion can thus be drawn about the impact of adjuvant treatments.
In conclusion, in our experience surgical resection provided cure in all the patients with benign tumors; in the presence of histologic characteristics of malignity cure may be achieved in the great majority of cases. As completeness of resection is probably the best prognostic factor, every LFTP must be considered as possibly malignant. Extended resection must be performed if any doubt about the completeness of resection exists.
References
England D.M., Hochholzer L., McCarthy M.J. Localized benign and malignant fibrous tumours of the pleura. A clinicopathologic review of 223 cases. Am J Surg Pathol 1989;13:640��.[Medline]
Cardillo G., Facciolo F., Cavazzana A.O., Capece G., Gasparri R., Martelli M. Localized (solitary) fibrous tumours of the pleura: an analysis of 55 patients. Ann Thorac Surg 2000;70:1808?.[Abstract/Free Full Text]
Khan J.H., Rahman S.B., Clary-Macy C., Kerlan R.K., Georges T.L., Hall T.S., Jablons D.M. Giant solitary fibrous tumor of the pleura. Ann Thorac Surg 1998;65:1461?.[Abstract/Free Full Text]
Hanau C.A., Miettinen M. Solitary fibrous tumor: histological and immunohistochemical spectrum of benign and malignant variants presenting at different sites. Hum Pathol 1995;26:440��.[Medline]
Suter M., Gebhard S., Boumghar M., Peloponisios N., Genton C.Y. Localized fibrous tumours of the pleura: 15 new cases and review of the literature. Eur J Cardiothorac Surg 1998;14:453��.[Abstract/Free Full Text]
de Perrot M., Kurt A.M., Robert J.H., Borisch B., Spiliopoulos A. Clinical behavior of solitary fibrous tumors of the pleura. Ann Thorac Surg 1999;67:1456?.[Abstract/Free Full Text]
Versluis P.J., Lamers R.J. S. Localized pleural fibroma: radiological features. Eur J Radiol 1994;18:124��.[Medline]
Moat N.E., Teale J.D., Lea R.E., Matthews A.W. Spontaneous hypoglycaemia and pleural fibroma: role of insulin like growth factors. Thorax 1991;46:932��.[Abstract]
Briselli M., Mark E.J., Richard Dickersin G. Solitary fibrous tumors of the pleura: eight new cases and review of 360 cases in the literature. Cancer 1981;47:2678?.[Medline]
El-Naggar A.K., Ro J.Y., Ayala A.G., Ward R., Ordoñez N.G. Localized fibrous tumor of the serosal cavities: immunohistochemical, electron-microscopic, and flow-cytometric DNA study. Am J Clin Pathol 1989;92:561��.[Medline]
Rena O., Filosso P.L., Papalia E., Molinatti M., di Marzio P., Maggi G., Oliaro A. Solitary fibrous tumour of the pleura: surgical treatment. Eur J Cardiothorac Surg 2001;19:185��.[Abstract/Free Full Text]
Okike N., Bernatz P.E., Woolner L.B. Localized mesothelioma of the pleura. J Thorac Cardiovasc Surg 1978;75:363��.[Abstract]
Dixon W.J. BMDP statistical software. Berkeley, CA: University of California Press, 1981.
Nagahiro I., Andou A., Aoe M., Sano Y., Date H., Shimizu N. Pulmonary function, postoperative pain and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362��.[Abstract/Free Full Text]
Battifora H., McCaughey W.T. Tumors of the serosal membranes. Washington, DC: American Registry of Pathology, Armed Forces Institute of Pathology, 1995.
|